Surgical and respiratory face mask
Star Care Face Mask Ultra FFP2 NR D 10 Units
€15.69
Shipping in 24-72h
Xsecure mask case with dark grey hanger
€3.58
Shipping in 24-72h
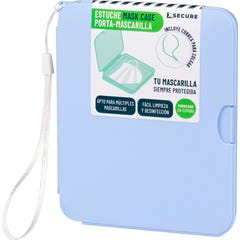
Xsecure Mask Case with Mint Green Hanger
€2.74
Shipping in 24-72h
Suavinex Mask Sv 6-10 years Dinos 2Ud
€11.81
Shipping in 24-72h
Endless Safe Mask Coral 1 mask + 2 filters
€5.83
Shipping in 24-72h
Lite Touch Diamond Children's Mask 1pc
€16.01
Shipping in 24-72h
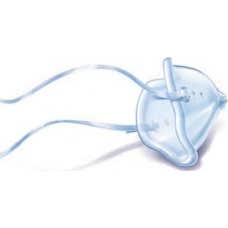
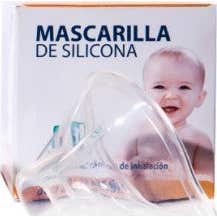
Pediatrics Healthcare Silicone Mask 0-18m Krt-r-i
€11.43
Shipping in 24-72h
Lite Touch Diamond neonatal mask 1pc
€12.98
Shipping in 24-72h
Inca Farma Adjustable mask hanger pink 1pc
€20.09
Shipping in 24-72h
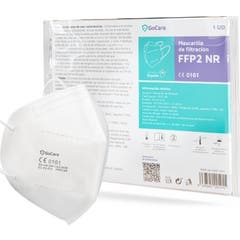
GoCare NR FFP2 Filtering Face Mask 1 Unit
€1.96
Shipping in 24-72h